Water is essential to sustain life on earth. Access to safely managed drinking water in a way that it’s available when needed and is free of contamination is not a given to many communities around the world. About two-thirds of our planet’s surface is covered in ocean waters, but this water cannot be consumed due to its high salt content. If we could remove salt from the seawater, through a process called desalination, we could harness the sea to solve the freshwater crisis. Desalination technologies indeed have been developed and some are in use as we speak—such as the RO (reverse osmosis) water filters used in homes and offices. However, these devices cannot handle seawater, which has a much higher salt content. Researchers have been working to develop better desalination technologies--methods that could match the challenge that seawaters present. “Existing technologies including reverse osmosis, electrodialysis and capacitive deionization are highly energy demanding or are prone to contaminating desalinated water with chemicals used in the desalination process,” says Dr. Musthafa Ottakam Thotiyl laying out one of the problems he is trying to address along with his research group at IISER Pune. A new method to desalinate salty water In this paper in the journal Joule, Dr Musthafa’s group reports a method to desalinate saline (salty) water during electricity generation without contaminating the desalinated water. For this, the team developed an electrochemical neutralization device, in which by using hydrogen redox reactions, they directly interconverted the energy derived from acid-base neutralization as electricity. The process involves only gases, water, protons (H+) and hydroxide ions (OH-) without a net redox, such that the products and the reactants of the reaction do not contaminate the desalinated water. The team has observed that about 13.6 kJ of energy is utilized for the removal of 1 mol of salt (sodium chloride) from saline water.
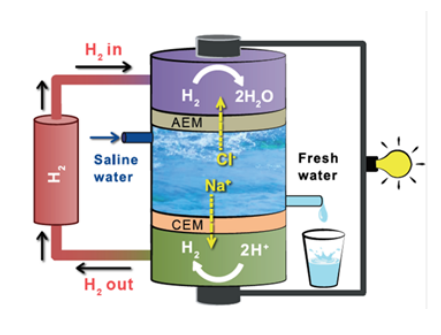 |
| Dr Musthafa's research group demonstrated that this electrochemical neutralization cell can spontaneously desalinate saline water during electric power production. CEM stands for cation (positive ion) exchange membrane and AEM for anion (negative ion) exchange membrane (Image: Dr Musthafa). |
Capitalizing on an acid-base neutralization reaction When an acid and a base are added together, they combine through a chemical process which produces water and releases heat. Electrochemically, if one wants to harvest this heat as electricity, the direct acid-alkali reaction has to be decoupled by an ionically conducting membrane, which will allow the neutralization reaction to proceed via electron movement through an external wire. This system is generally referred to as an electrochemical device and serves to convert chemical energy into electrical energy. However, for harvesting the neutralization energy directly as electricity, the challenge is to identify a reaction sequence without a net redox. Any net redox reaction will be additional to the acid-base neutralization pathway, and hence threatens to contaminate the neutralization pathway. Dr Musthafa and his team realized this direct inter-conversion of neutralization energy as electricity via hydrogen redox without a net redox reaction. As shown in the image, hydrogen molecules are consumed at the anode and regenerated at the cathode. There is no net consumption of the hydrogen molecules during the operation of the device. This allows a direct harvest of the neutralization energy as electricity without a net redox reaction. The thermodynamics of the neutralization pathway The parameter of thermodynamic efficiency (TE) tells us how an electrochemical cell achieves energy conversion. Most state-of-the-art electrochemical energy devices give off heat to the surroundings during electricity production, which results in the thermodynamic efficiency less than one. For the electrochemical cell developed by Dr Musthafa’s group, the TE efficiency is ~1.43. The direction of heat flow in an electrochemical system is decided by a thermodynamic property called entropy, a measure of the degree of disorder in a system. Energy will be lost as heat from the electrochemical cell to the surroundings during electricity generation if entropy change is negative and vice versa. “Most fuel cells have a negative entropic heat with TE less than one. Notable exceptions are formic acid fuel cell and the carbon-carbon monoxide fuel cell, where TE is >1 and the devices are used to cool the surroundings. The neutralization pathway that we demonstrate in this paper shows a positive entropic heat, such that nearly 30% of the total energy is harvested from the surroundings. And this results in a TE greater than 1. This device in a 3-compartment configuration allows removal of salt without contaminating the water,” explains Dr Musthafa. He notes that this electrochemical neutralization cell offers various opportunities in the sustainable energy landscape such as electro-organic synthesis, fuel separation, fuel reforming, and rechargeable aqueous batteries, all of which are currently being investigated in their laboratory. The group acknowledges funding from Government of India’s DST, SERB, DST-Nanomission, and CSIR. Article Citation: An electrochemical neutralization cell for spontaneous water desalination. Zahid Manzoor Bhat, Deepraj Pandit, Shane Ardo, Ravikumar Thimmappa, Alagar Raja Kottaichamy, Neethu Christudas Dargily, Mruthunjayachari Chattanahalli Devendrachari, and Musthafa Ottakam Thotiyl (2020). Joule DOI:https://doi.org/10.1016/j.joule.2020.07.001. - With inputs from Dr Musthafa O.Thotiyl; edited by Shanti Kalipatnapu






